This research interest focuses on hydrogen and nitrogen isotopes in (1) organic matter through diagenesis and thermal maturation of source rocks, and in (2) bacterially produced coalbed methane. Our projects have been funded by the U.S. Department of Energy, Basic Energy Sciences, and by the Petroleum Research Fund. The following sections highlight a few selected key aspects of our research:
Hydrogen and Nitrogen Isotopes in Fossil Fuels
Introduction to the concept of exchangeable hydrogen
The original isotopic composition of organic matter relates to the environment of biosynthesis, thus principally permitting the use of stable isotopes of fossil organic matter for paleoenvironmental reconstruction. However, changes in the stable isotopic composition occur after the death of organisms, especially upon heating of organic matter in sediments at greater depth. The H- and N-isotopic interactions (= in part exchange, in part irreversible chemical transfer; see figure below) between (1) organic hydrogen and inorganic hydrogen from water (https://dx.doi.org/10.1016/S0016-7037(99)00221-5) and (2) between organic nitrogen and inorganic dissolved ammonia-nitrogen (Schimmelmann et al., 1998, American Chemical Society Symposium Series 707:226-242) were demonstrated in hydrous pyrolysis experiments and in 5-year heating experiments at temperatures as low as 100 °C. In all cases, large isotopic transfer between water-derived hydrogen and organic hydrogen occurred during heating of immature source rocks.
See Figure below: Hypothetical kerogen molecule visualizing important isotopic transfer mechanisms for organic hydrogen. (A) Reversible exchange of organic H bound to O, N, or S in functional groups with H2O, even at low temperature. This pool of exchangeable organic H is also called labile, or active, and is found in many substrates. (B) Ionic exchange of organic H in a-position to C=O and COOH via enolization. This exchange is reversible under suitable pH and temperature conditions. (C) Ionic exchange of aromatic H, and of H bound to tertiary C atoms. Theses exchanges are reversible under suitable pH and temperature conditions. (D) Irreversible H-transfer via radical reactions that may proceed as chain reactions affecting several H-sites in one or more molecules. (E) The largest pool of isotopically conservative (= least affected by H-exchange or transfer) H is located in alkyl groups.

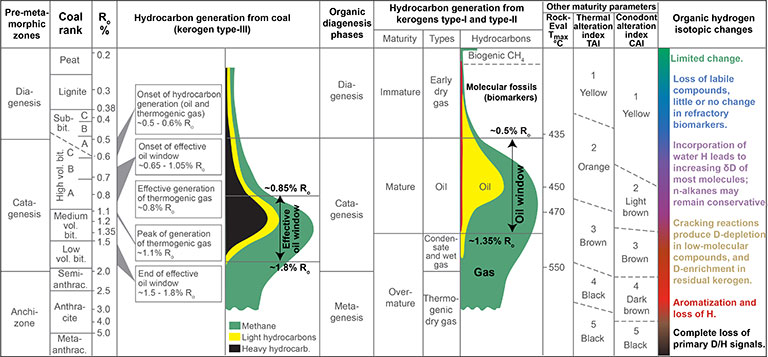
The effect of increasing thermal maturity on organic hydrogen stable isotope ratios:
Changes in the D/H ratio of sedimentary organic matter (SOM) during thermal maturation have been difficult to interpret because the effects of hydrogen exchange and kinetic fractionations are confounded in natural samples. Recent analytical developments have dramatically improved our understanding of the responsible mechanisms. In 2006 we reviewed experimental and field data that document a progressive increase in the D/H ratio of most organic H at the bulk and molecular levels, and suggest that the transfer of H from water to organic matter is the most important mechanism leading to those changes.
Nevertheless, δ2H values of organic hydrogen can preserve quantitative information about paleoclimate throughout diagenesis, and some qualititative information through catagenesis.
See Figure below: Relationships between metamorphic zones, coal rank, diagenetic and maturity phases, hydrocarbon generation, and organic δ2H changes.
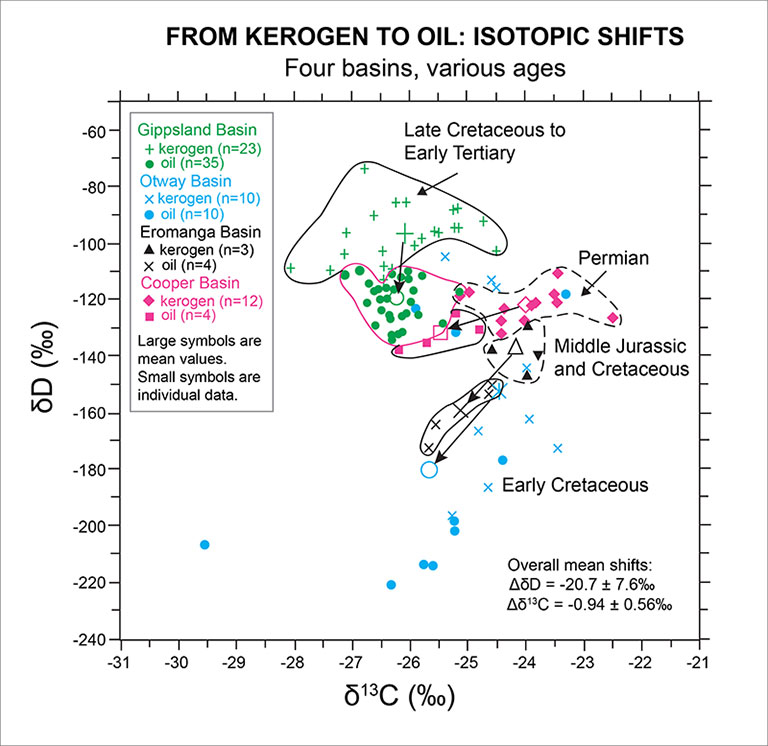
Source rocks, oils, and oil fractions
Natural kerogens of different type and maturity, and a large matrix of oils, fractions of these oils, and associated formation waters were isotopically characterized to assess the extent of natural hydrogen isotopic transfer during maturation and storage over geologic time. We analyzed kerogens and oils from four different basins for which we have significant understanding of the source rock paleogeography, organic inputs, and geologic history to safely assume that the oils derive from distinct source rocks with well-known source ages (see Figure below). Permian kerogens and oils from the Cooper Basin, Middle Jurassic samples from the Eromanga Basin, and Late Cretaceous/Early Tertiary samples from the Gippsland Basin all plot in quite distinct isotopic ranges for each basin, with a uniform trend toward more negative δ2H values and δ13C values from kerogen to oil. The mean δ-values of Early Cretaceous kerogens and oils from the Otway Basin show a similar shift, although the individual data for kerogens and oils are spread over much larger ranges and thus indicate significant heterogeneity within Otway Basin source rocks. The overall mean isotopic shifts for the four basins are δ2Hoil-ker = -20.7 ± 6.6 ‰ and δ13Coil-ker = -0.94 ± 0.56 ‰.
See Figure below: δ2H and δ13C values of kerogens and oils from four different basins, each with a distinct source rock age: Cooper Basin (Permian), Eromanga Basin (Middle Jurassic), Otway Basin (Early Cretaceous), and Gippsland Basin (Late Cretaceous/Early Tertiary). Small symbols indicate individual isotopic results, whereas large symbols represent basin-specific mean δ-values. For the Cooper, Eromanga, and Gippsland basins, dashed contour lines indicate the isotopic ranges of kerogens, and solid lines help visualize the isotopic ranges for oils. No ranges are marked for the Otway Basin, because δ-values of kerogens and oils are spread over large ranges and thus indicate heterogeneity of Otway Basin source rocks.
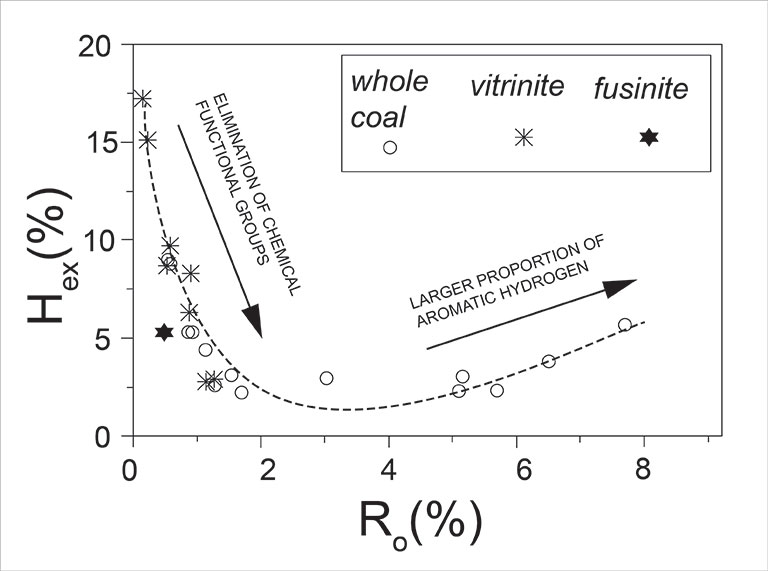
Exchangeable hydrogen in thermally maturing kerogen
Organic hydrogen isotopic exchangeability (Hex) and δ2Hn values of non-exchangeable organic hydrogen in kerogen from coals have been investigated spanning thermal maturation levels from lignite to graphite. Hex in coal depends on thermal maturity and maceral composition. The relative abundance of Hex is highest in lignite with about 18 % of total hydrogen and decreases to around 2.5 % in coals with Ro of 1.7 to ca. 5.7 %. Reversing this trend, at higher rank (Ro above 6 %) Hex increases slightly, in spite of the fact that the abundance of total organic hydrogen decreases. Hex of kerogen from bulk high volatile bituminous coal tends to be smaller than Hex of the vitrinite kerogen from the same coal, whereas kerogen from the fusinite fraction has substantially lower Hex. δ2Hn in kerogen is influenced by original isotopic differences from the various source materials and by isotopic interaction with water during thermal maturation, and therefore δ2Hn does not show an overall consistent trend with maturity. A trend toward more negative δ2Hn values is revealed, however, for Illinois Basin and Appalachian Basin coals with increasing maturity from Ro of 0.55 % to 1.4 %. In most cases, δ2Hn values in kerogens from whole coal samples are similar to those in kerogens from the respective vitrinite fractions.
We conclude that a directly measured hydrogen stable isotope ratio of bulk coal, even after careful demineralization of the coal, reflects (i) the maceral composition, (ii) the history of coal-water isotopic interaction along thermal maturation of organic matter, and (iii) the presence of isotopically exchangeable organic hydrogen. The latter complication can be mitigated by isotopic double-equilibration of exchangeable hydrogen.
Quantification of hydrogen isotopic exchangeability in coal and its isolated macerals offers an analytically independent proxy for assessing organic-geochemical processes along thermal maturation.
See Figure below: The isotopic exchangeability of organic hydrogen (Hex, in % of total organic hydrogen) in kerogens with water hydrogen shows a distinct relationship with vitrinite reflectance (Ro), or with thermal maturity.
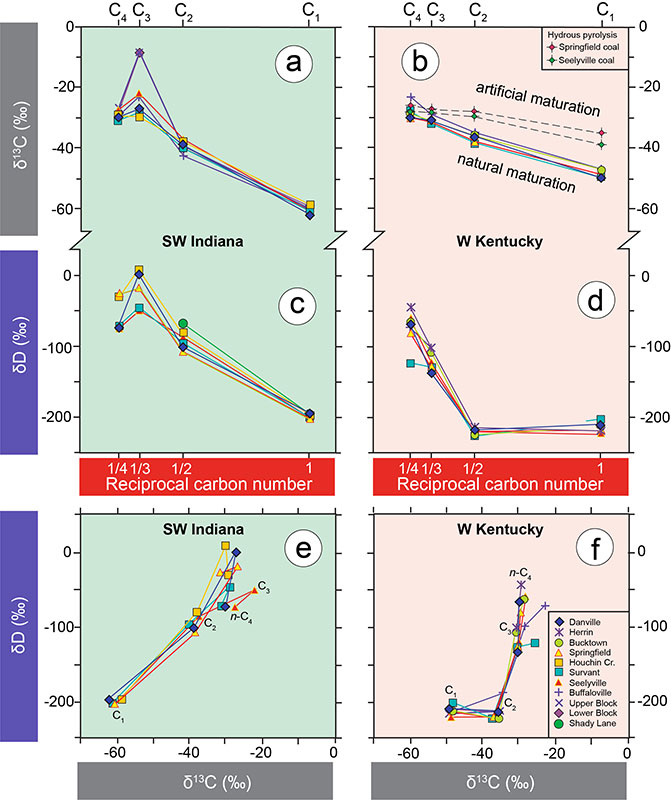
Hydrogen stable isotope ratios in coalbed gases
Coalbed gases and waters from exploratory and production gas wells in the southeastern Illinois Basin were sampled to assess geochemically the origin of coalbed gases, with an emphasis on the Springfield and Seelyville Coal members that are commercially targeted for coalbed methane production in Indiana. On-line analyses of hydrocarbon gases methane to butanes (C1, C2, C3, n-C4, i-C4) and CO2 yielded gas concentrations, plus δ2H and δ13C values. The low thermal maturity of Indiana coals with vitrinite reflectance R0 ~ 0.6 % is in agreement with an overwhelmingly biogenic isotopic signature of coalbed gases containing ≥96 % methane generated via bacterial CO2-reduction. In contrast, thermogenic gas was generated in the stratigraphically equivalent coal beds in western Kentucky’s Rough Creek Graben zone where higher maturities of up to R0 ~ 0.8 % were reached owing to tectonic and hydrothermal activity. No secondary biogenic methane was observed in more mature western Kentucky coal beds where greater burial depth limits the recharge of meteoric water.
Biogenic and thermogenic coalbed gases represent two end-members that are compositionally and isotopically distinct. Microbial biodegradation of thermogenic C2+ hydrocarbon gases in Indiana coal beds preferentially targets C3 and introduces isotope fractionation whereby remaining C3 is enriched in deuterium and 13C.
See Figure (right): Compound-specific isotopic data for Indiana and Kentucky coalbed gases. (a) and (b) show δ13C values, and (c) and (d) show δ2H values of hydrocarbons plotted against the reciprocal carbon numbers of methane to butane according to Chung et al. (1988). The same δ13C and δ2H data are cross-plotted for Indiana (e) and western Kentucky (f).